Potential Mechanisms of BBB Disruption in Migraine
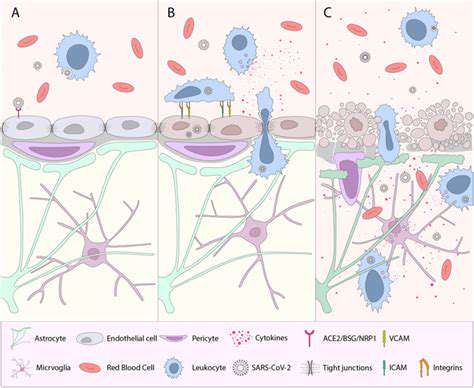
Potential Mechanisms of Inflammatory BBB Disruption
Inflammatory processes represent one of the primary pathways for blood-brain barrier compromise. When inflammation occurs, it initiates a domino effect of biochemical events that gradually weaken the barrier's structural integrity. This breakdown allows substances that normally remain in the bloodstream to penetrate brain tissue, potentially causing neurological dysfunction. Researchers are particularly interested in identifying the specific molecular pathways involved, as these could become targets for future therapies.
The inflammatory response involves numerous signaling molecules including cytokines, chemokines, and reactive oxygen species. These substances can alter the behavior of the endothelial cells forming the BBB, causing them to loosen their tight junctions. Additionally, they stimulate production of matrix metalloproteinases—enzymes that degrade the extracellular matrix supporting the BBB's structure.
Role of Oxidative Stress in BBB Dysfunction
Oxidative stress occurs when the body's antioxidant defenses are overwhelmed by reactive oxygen species. In the context of the BBB, this imbalance can be particularly damaging. Excessive oxidative molecules can directly injure the endothelial cells and pericytes that constitute the blood-brain barrier, compromising its protective function. This damage often manifests as increased permeability, allowing potentially harmful substances to enter the brain environment.
What makes oxidative stress particularly problematic is its ability to trigger inflammatory responses, creating a destructive feedback loop. As inflammation generates more oxidative stress, which in turn worsens inflammation, the BBB's integrity continues to deteriorate. This vicious cycle may contribute to the progression of various neurological conditions.
The Impact of Cytokines on BBB Permeability
Cytokines serve as the body's chemical messengers during immune responses, but some varieties can significantly affect BBB function. Pro-inflammatory cytokines like TNF-α and IL-1β have been shown to directly impair endothelial cell function and disrupt the tight junctions between them. When these junctions fail, the barrier becomes more porous, permitting substances that normally remain excluded from the brain to enter.
Beyond their direct effects on endothelial cells, cytokines also promote the expression of adhesion molecules. These surface proteins facilitate the migration of immune cells across the BBB, potentially exacerbating neuroinflammation. This dual action makes cytokine regulation an important area of research for neurological disorders.
The Contribution of Astrocytes and Microglia
The brain's resident support cells—astrocytes and microglia—play complex roles in BBB maintenance and dysfunction. Under normal circumstances, these glial cells help maintain the barrier's integrity. However, when activated by pathological conditions, they can contribute to its breakdown. Microglia, the brain's primary immune cells, release inflammatory mediators that can damage the BBB when chronically activated.
Astrocytes, which normally provide structural and metabolic support to neurons and blood vessels, can also contribute to BBB dysfunction when improperly activated. Their processes surround cerebral blood vessels and help maintain barrier properties, but inflammatory conditions can cause them to release factors that impair BBB function instead.
The Role of Matrix Metalloproteinases (MMPs)
Matrix metalloproteinases represent a family of enzymes capable of degrading various components of the extracellular matrix. When MMP activity increases—often in response to inflammation—it can significantly weaken the structural foundation supporting the BBB's endothelial cells. This enzymatic activity essentially dissolves the glue holding the barrier together, leading to increased permeability.
Because inflammation frequently triggers MMP production, these enzymes serve as a key link between inflammatory processes and BBB dysfunction. Pharmaceutical approaches that target specific MMPs might offer therapeutic potential for conditions involving BBB breakdown.
Mechanisms of Mechanical Stress on the BBB
Physical forces can also impact the blood-brain barrier's integrity. Sustained exposure to high shear stress from blood flow can physically damage the delicate endothelial cells lining cerebral blood vessels. This mechanical stress tends to be most pronounced in areas where blood flow patterns create turbulent conditions or particularly high velocities.
Changes in cerebral blood flow dynamics—whether from vascular abnormalities or systemic blood pressure fluctuations—can similarly stress the BBB. Understanding how these mechanical factors contribute to barrier dysfunction may lead to new approaches for protecting the BBB in various clinical scenarios.
Therapeutic Implications and Future Directions
Therapeutic Implications of Blood-Brain Barrier Dysfunction in Migraine
The growing understanding of BBB dysfunction in migraine pathophysiology opens exciting possibilities for treatment innovation. Developing interventions that specifically target the BBB's compromised integrity could revolutionize migraine management. Potential approaches might include therapies that actively repair barrier function or at least mitigate the consequences of its disruption. Such treatments could address the root causes of migraine rather than just alleviating symptoms.
As research continues to elucidate the precise mechanisms linking BBB dysfunction to migraine, more specific drug targets will likely emerge. This precision medicine approach could yield treatments with fewer side effects and greater efficacy than current options. The challenge lies in translating these scientific insights into clinically viable therapies.
Targeting Specific BBB Components for Migraine Treatment
The BBB's complex cellular composition offers multiple potential intervention points. Each component—endothelial cells, pericytes, and astrocytes—contributes uniquely to barrier function and could be targeted therapeutically. For instance, if research identifies specific proteins in endothelial cells that are particularly vulnerable in migraine, drugs could be developed to protect or stabilize these molecules.
Developing Novel BBB-Targeted Therapies
Current migraine treatments primarily focus on symptom management through vasoconstriction or pain modulation. Future therapies might instead aim to restore normal BBB function, potentially preventing migraine attacks before they start. Innovative approaches could include biologics that strengthen intercellular junctions or nanotechnology-based delivery systems that repair damaged barrier components.
Acute Migraine Treatment Strategies
For acute migraine attacks, rapid-acting therapies that address BBB dysfunction could provide more effective relief. Such treatments might work by quickly stabilizing the barrier or interrupting the inflammatory cascade that leads to pain. Combining these with existing acute therapies could offer more comprehensive attack management.
Preventive Strategies and Long-Term Management
Chronic BBB impairment may contribute to migraine chronification, making barrier protection an important preventive strategy. Regular treatments that maintain BBB integrity could potentially reduce attack frequency and severity over time. This approach might be particularly valuable for patients with frequent or treatment-resistant migraines.
Future Directions and Research Priorities
Significant research gaps remain in understanding BBB dysfunction in migraine. Key priorities include elucidating the temporal relationship between BBB changes and migraine attacks, identifying biomarkers of barrier dysfunction, and developing reliable animal models. Multidisciplinary collaboration will be essential to translate basic science discoveries into clinical applications.
Translational Research and Clinical Applications
Bridging the gap between laboratory findings and patient care represents the ultimate challenge. Preclinical studies using appropriate animal models must carefully evaluate potential therapies before human trials. Clinical researchers will need to develop sensitive methods for assessing BBB function in migraine patients to properly evaluate new treatments.