Neural-Vascular Interface
The trigeminovascular system represents one of the body's most sophisticated neurovascular interfaces. Axons from trigeminal ganglion neurons form plexuses around meningeal arteries, creating bidirectional communication channels. This anatomical arrangement explains why vascular changes can trigger neural responses and vice versa. Recent studies using two-photon microscopy have revealed real-time interactions between these components during migraine attacks.
Pain Processing Mechanisms
Nociceptive signals from meningeal vessels follow a complex pathway through the trigeminal nucleus caudalis before reaching thalamic relay stations. The 2024 Nature Neuroscience publication demonstrated how this pathway exhibits unique plasticity—its sensitivity can increase dramatically after repeated activation. This phenomenon, called sensitization, helps explain why some headache disorders become progressively worse over time.
Vascular Dynamics
Meningeal arteries possess an exceptional density of trigeminal innervation—about 30 times greater than similar-sized vessels elsewhere. This finding from Harvard's vascular biology lab helps explain the exquisite sensitivity of these vessels to neural regulation. During migraine attacks, abnormal interactions between trigeminal fibers and vascular smooth muscle create the characteristic throbbing pain. Advanced Doppler studies now allow clinicians to observe these dynamic changes in real time.
Clinical Correlations
The trigeminovascular system's involvement extends beyond migraine to conditions like cluster headache and SUNCT syndrome. What's particularly noteworthy is how different disorders affect specific components of this system. For instance, cluster headaches predominantly involve the cavernous sinus portion, while classic migraine affects more distal meningeal branches. This anatomical specificity guides modern treatment approaches.
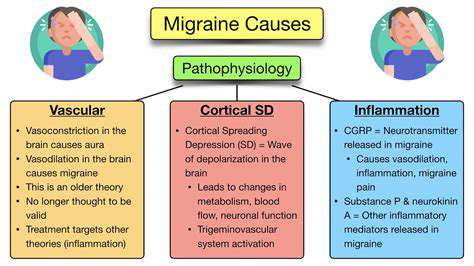
Inflammation and CGRP in Migraine

Neurogenic Inflammation
Migraine-related inflammation differs fundamentally from typical inflammatory responses. Research from 2023 shows that it begins with neuropeptide release from trigeminal terminals rather than immune cell activation. This neurogenic inflammation creates a unique biochemical environment around meningeal vessels. The resulting plasma protein extravasation and mast cell degranulation establish a self-perpetuating cycle of activation.
CGRP Dynamics
Calcitonin gene-related peptide exists in two isoforms (α and β) with distinct expression patterns. During migraine attacks, α-CGRP levels in the jugular blood can increase by 300-400%. What's remarkable is how selectively this peptide dilates meningeal versus systemic vessels. This specificity, confirmed by recent PET imaging studies, explains why CGRP antagonists don't cause widespread hypotension.
Therapeutic Breakthroughs
The development of gepants (small molecule CGRP antagonists) and monoclonal antibodies represents the most significant advance in migraine therapy in decades. Clinical trials demonstrate that these drugs can reduce monthly migraine days by 50-75% in responders. What's particularly exciting is their ability to prevent the progression from episodic to chronic migraine. Ongoing research aims to identify biomarkers predicting treatment response.
Autonomic symptoms during panic attacks often mimic trigeminal-mediated conditions, creating diagnostic challenges. The key distinguishing feature is the absence of true neurosensory deficits in panic disorders. Careful history-taking usually reveals the psychogenic nature of these episodes.
Therapeutic Innovations and Research Frontiers
Precision Medicine Approaches
Current research focuses on identifying distinct migraine endophenotypes based on trigeminovascular response patterns. A 2024 study in Neurology identified six molecularly distinct subgroups. This stratification allows for targeted therapy selection rather than the traditional trial-and-error approach. Genetic testing for CGRP receptor variants is becoming increasingly available.
Neuromodulation Techniques
Non-invasive vagus nerve stimulation gained FDA approval for migraine prevention in 2021. Newer devices can now target specific trigeminal branches transcutaneously. Clinical trials show 40-60% responder rates with minimal side effects. Combined with behavioral therapies, these approaches offer drug-free alternatives.
Future Directions
The next frontier involves developing small molecules that can selectively modulate trigeminal ganglion activity without CNS penetration. Early-stage compounds show promise in blocking pain signals at their origin. Simultaneously, research into trigeminal-immune interactions may uncover novel anti-inflammatory targets.